I think it's high time we start profiling a few applications that have been approved for use by the FDA, discuss their functionality, and potentially profile the impending issues they may encounter.
Today's profile will be on the iBG Star application and plug-in device, cleared as a glucose monitor: 2010 press release, 2011 FDA clearance press release, and the 510(k) summary from the FDA's database.
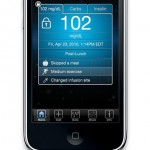
I downloaded the app to navigate the user interface of the tool. It allows you to log entries with Glucose (by mg/dL), Carbs (in grams), and units of insulin. The "data" tab of the application allows the user to view trend charts, log measurements in an electronic log book, and a statistics page that will calculate the average and standard deviation of glucose measurements overall, and at various points in the day. The application also includes a "share" functionality to send data, and an "info" tab to adjust settings or get help.
So iBG Star has reached the promised land - cleared by FDA, glucose app for iPhone and Android, aesthetic and intuitive user interface. They're set, right? Unfortunately, it's not that easy.
Firstly, the product was cleared under four different FDA product codes, meaning the regulatory department has to keep on top of regulatory news and changes for four different "products". Add to that, they're a mobile medical application, resulting in even further surveillance of that market and the changing regulations. Add to that, their design engineers were probably sent into a tailspin when Apple announced the change from a 32 pin connector to the new 'Lightning' connector on the new iProducts (iPhone, iPod touch, iPad, etc.). Thankfully for the developers, the Android device manufacturers will likely continue to use the microUSB connector.
Finally, notice the timing of the two press releases linked above. The product was announced (and the 510(k) is dated) in 2010, but the release did not occur until 2011. The manufacturer will still need to consider those types of time-frames when making significant improvements on the technology which require re-submittal.
Is iBG Star actually falling? No, because they have cleared significant hurdles in getting the product cleared. The manufacturer's ability to stay abreast of changing technology and regulation will determine whether they shine for eons, or simply fall to Earth.
-RTK
Image Credits: Merlin2525 at OpenClipArt.org