RQM+ Live! #23 —
Recorded 19 November, 2020
Upcoming and on-demand education, commentary from thought leaders, Q&A features, and more.
Make real world evidence work for you: How to leverage real world data in the US and EU
---
RQM+ Live! is a biweekly and interactive live show featuring expert panelists discussing timely topics, challenges, and solutions in the medical device industry. The panelists also answer audience questions. Every episode is added to our podcast, too.
---
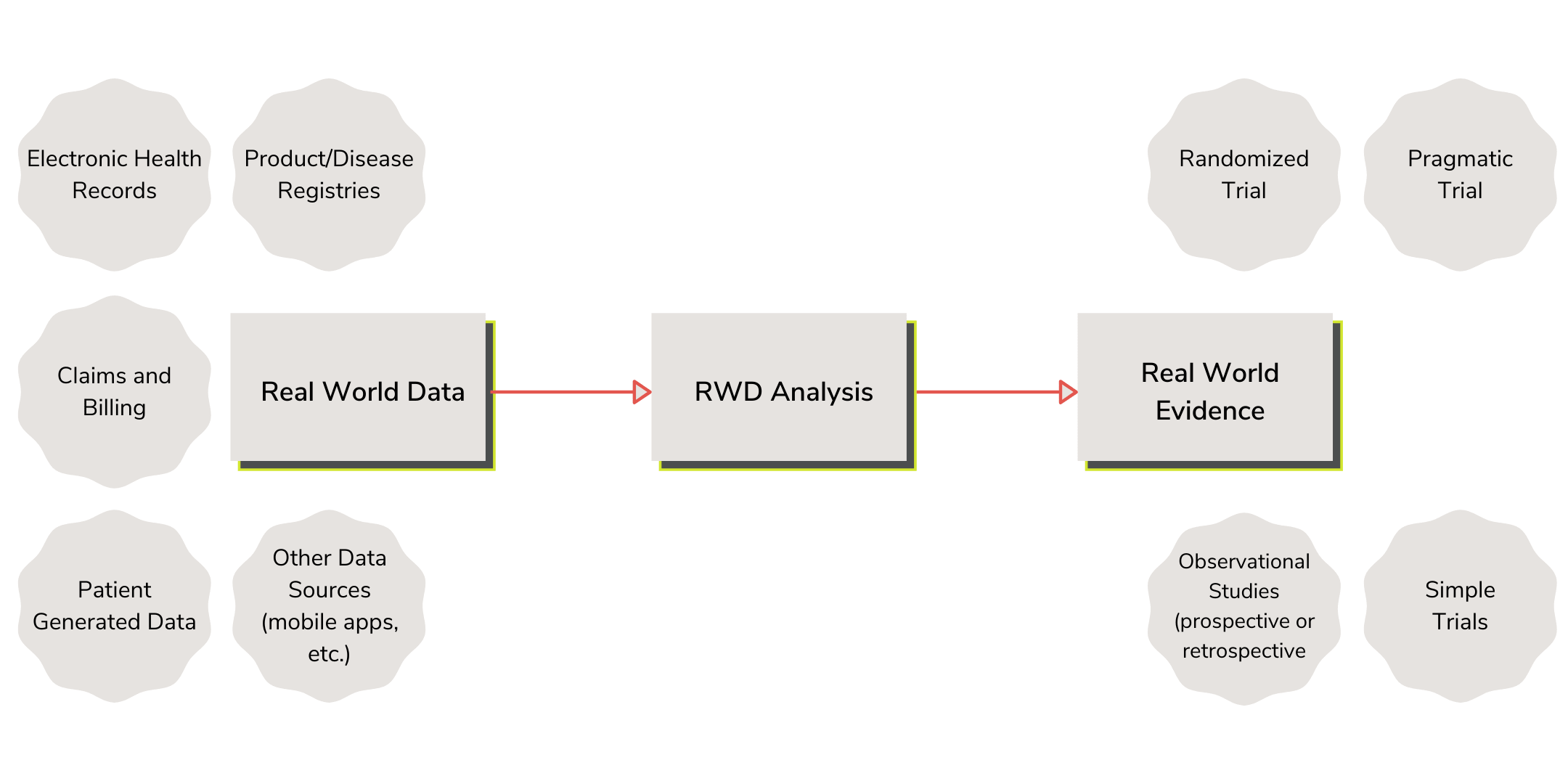
As defined by the FDA, Real-world data (RWD) are the data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources, like electronic health records and product and disease registries. With the additional requirements of the EU MDR, RWD can also be gathered from PMCF studies and surveys, and EUDAMED. Real-world evidence (RWE) is the clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of RWD. RWE can be generated by different study designs or analyses, including but not limited to, randomized trials, including large simple trials, pragmatic trials, and observational studies (prospective and/or retrospective).
More simply put, RWD are the data sources obtained outside of a traditional clinical trial, which are then analyzed to generate RWE or used to define a study to create RWE. RWE can provide a faster and more efficient method to meet the evidentiary requirements for FDA and EU regulatory submissions, but it is not without its challenges.
Determining what RWD and RWE are and how to apply them for your device can be daunting. In this show, we will simplify these terms and discuss best practices. We'll also answer the questions we often receive including the following:
-
Real world data is often messy as much of it comes from uncontrolled use of the device, so how do I filter out the noise to gain supportive information
-
How can I leverage all the PMCF data I need to collect for EU to support an expanded indication in the U.S.? Conversely, how can I leverage FDA RWE to meet my PMCF requirements?
-
Would you recommend a pre-submission meeting before collecting RWE to support a new indication?
-
What happens if I collect RWE and find a problem – either off-label use or unanticipated adverse events?
Bring your toughest RWD/RWE questions for our experts to answer LIVE!
-
Amie Smirthwaite, Ph.D. – Head of Global Clinical Practice, Maetrics (recent Head of Global Clinical at BSI)
-
Nancy Morrison – Exec. Director, Regulatory and Quality Consulting Services, and leader of R&Q EU MDR Leadership Council, R&Q
-
Jon Gimbel, Ph.D. – Executive Director, Regulatory and Quality Consulting Services, and technical leader of R&Q CER/PER team, R&Q
-
Celeste Maksim – Principal Consultant, R&Q
-
Kevin Go – Senior Engineer (former FDA CDRH Lead Reviewer), R&Q