Post-market surveillance deliverables for medical devices vary depending on the market. Our global team has experience with every type of device and every regional regulation. Whether you are launching a new product, transitioning to EU MDR, or need a correction plan, our team is here to help.
We know that data from post-market surveillance is just part of the story and understand the importance of accurate data flow between PMS, clinical evaluation reports, and risk management systems. We’ll create a strategy for integrating all of your systems and help you successfully implement it.
Our global team is comprised of engineers and scientists who understand how medical devices work, how performance is measured, and what type of documentation and evidence is required. With former regulators and notified bodies on staff, we have unique insights into how regulatory bodies and reviewers are likely to respond.
We’ll help align your internal teams and put systems and timelines in place to meet submission deadlines. We know that notified bodies want to see the whole story, so we’ll develop methods for consistent data assessment, cross-functional review, and justification when drafting your PMCF plan and reports.
Learn more about Post-Market Clinical Follow-up
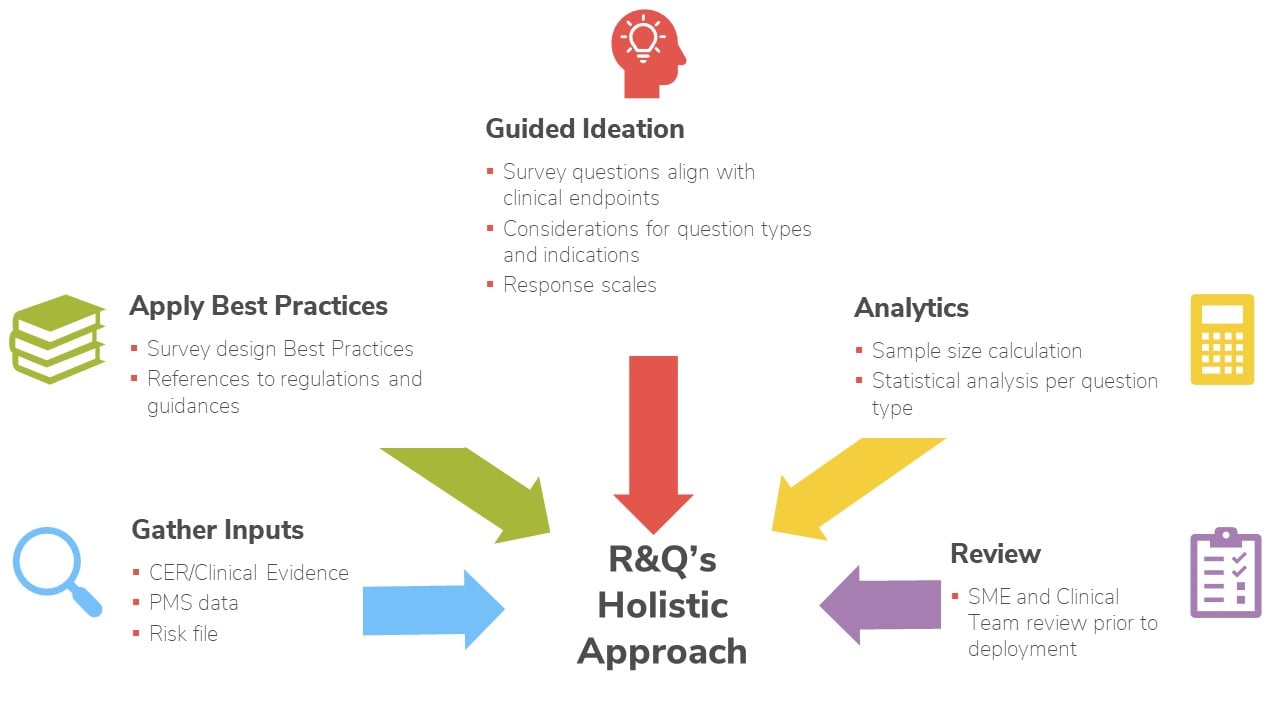
Developing and executing compliant user surveys requires technical expertise and time that not all manufacturers have. RQM+ provides the necessary resources—including people, processes, and technology— to gather the clinically meaningful and scientifically valid user surveys you need to prepare PMCF reports.
Whether you are creating your first PMCF plan or need to remediate documents with nonconformities, RQM+ uses a clinical evidence matrix to identify and fill gaps. The results are fewer questions from regulators, shorter review times, and clarity and consistency across all documentation.



.png)